
August 27, 2024 (ATLANTA) – Bruder Healthcare, a Hilco Vision Company, is pleased to announce that it has entered into an exclusive distribution agreement with Santen S.A. to market Eyeleve™ MGD* in the United States. To be marketed and distributed in Q2 2025 as Eyeleve MGD, this innovative preservative-free ophthalmic emulsion provides moisturization, lubrication, and protection for moderate to severe evaporative dry eye (EDE). With 80 percent of dry eye patients suffering from EDE, Eyeleve MGD will serve a crucial need of patients and practitioners alike.
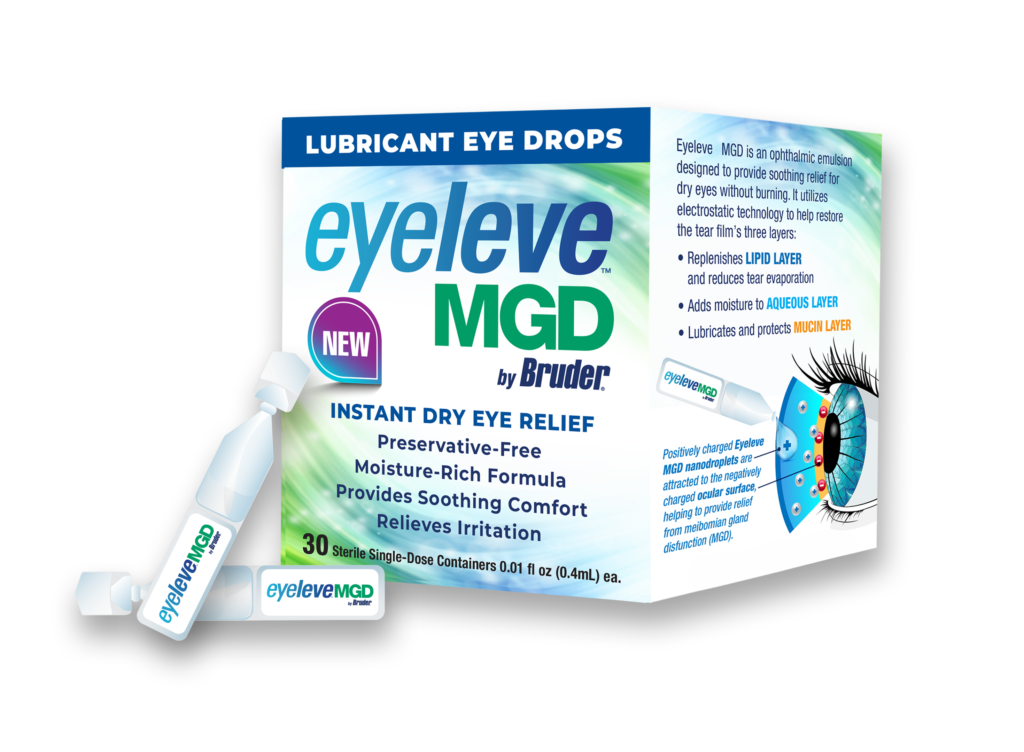
To be marketed and distributed in early 2025 as Eyeleve™ MGD
How it works
The formulation  uses a preservative-free oil-in-water cationic emulsion technology. This technology delivers ingredients through electrostatic attraction between positively charged droplets and the negatively charged ocular surface. This innovative approach promotes relief from dry eye symptoms by moisturizing, lubricating, and protecting the eyes.
uses a preservative-free oil-in-water cationic emulsion technology. This technology delivers ingredients through electrostatic attraction between positively charged droplets and the negatively charged ocular surface. This innovative approach promotes relief from dry eye symptoms by moisturizing, lubricating, and protecting the eyes.
Hilco Vision’s Brent Jones, Global Head of Vision Care Solutions, commented “We are thrilled to introduce Eyeleve MGD to our portfolio of dry eye treatments. Adding Eyeleve MGD strengthens our product line and better equips eye care professionals to provide solutions for patients on the road back to healthy eyes.”
Research-Backed
The hypo tonicity of the emulsion in Eyeleve MGD adds moisture by lowering the salt concentration of tears, while the lipid component lubricates and protects the surface of the eye. This unique combination of ingredients ensures that patients experience relief from dry eye symptoms, improving their overall eye health and comfort.
Bruder Healthcare and Hilco Vision are committed to delivering top-tier dry eye treatments to patients and practitioners. The upcoming launch of Eyeleve MGD in early 2025 reinforces this dedication. With innovative solutions like Eyeleve MGD, Hilco Vision continues to enhance its market-leading, comprehensive approach to dry eye care.
For more about Eyeleve MGD, visit eyeleve.com.
About Bruder Healthcare
Bruder Healthcare designs and markets therapeutic and ophthalmic medical products to professionals and consumers around the world, including the #1 doctor-recommended moist heat eye compress. Their broad line of proprietary products provides relief for the millions of people who suffer from pain, dry eye, and other ocular surface conditions. Bruder Healthcare is a part of the Hilco Vision family of eye care brands and companies. Open Your Eyes to Bruder® at bruder.com.
About Hilco Vision
Hilco Vision, a division of The Hilsinger Company Parent, LLC, is an industry leading global eyewear/eye care company delivering comprehensive solutions to customers, built on a platform of innovation and operational excellence. It has direct subsidiaries in the USA, Canada, UK, Germany, Australia, China, Hong Kong, Belgium and the Netherlands. The company’s product portfolio encompasses Prescription Safety, Lenscare, Eyewear Accessories, Lab & Dispensing, Ocular Surface, Exam Supplies, and Vision Testing.
For more information visit hilcovision.com.
REFERENCE:
*EYELEVE is a pending trademark application of The Hilsinger Company Parent, LLC (dba Bruder Healthcare).




